Alkanols And Alkanoic Acids – Formula And Properties
Alkanol and Alkanoic are one of the topics in organic chemistry that many science students want to know more about. And the reason is that there is no chemistry exam, that questions on them won’t come out.
So, this article we help you gain insight into the general formula, preparation, and properties of alkanol and alkanoic.
Alkanols
Aliphatic alkanols are compound in which hydroxyl groups are linked to alkyl groups. The hydroxyl group is the functional group of the alkanols as it is responsible for their characteristics chemical properties.
Alkanol Formula
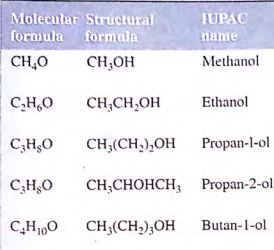
The general molecular formula of alkanol is CnH2n+1OH. Since the CnH2n+1 is the alkyl group and can be represented generally by R, the general formula of the monohydric alkanols can be written as ROH. The simplest member of the series is methanol (CH3OH).

Types of Alkanols
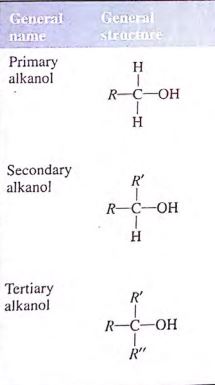
There are three types of alkanols, they are
- Primary alkanol
- Secondary alkanol
- Tertiary alkanol
A primary alkanol has only one alkyl group attached to the carbon atom that carries the hydroxyl group, a secondary alkanol has two, and a tertiary has three.
Preparation of Alkanols
For the preparation process, I will be making use of the preparation of ethanol in this article.
Ethanol can be prepared in the laboratory using the following methods
- Fermentation
- From ethene
- Hydrolyzing iodoethane with an alkali
From Ethene: ethene is obtained in large quantities by the cracking of petroleum. It is first absorbed in 95% tetraoxosulphate(VI) acid at 80 degree Celsius and 30 atm to form ethylhydrogentetraoxosulphate(VI). This is then hydrolyzed by boiling with water.
C2H4 (g) + H2SO4 (aq) -> C2H5HSO4 (aq)
C2H5HSO4 (aq) + H2O (l) -> C2H5OH (aq) + H2SO4 (aq)
The ethanol formed is distilled off, leaving the acid, which can be concentrated and used again.
Physical Properties of ethanol
- It is colourless, volatile liquid with a characteristic taste and smell
- it has no action on litmus
- it is readily soluble in water in all proportions
- it has a boiling point of 78 degree Celsius
Read: Balancing chemical equations with examples
Alkanoic
Alkanoic acids contain the carboxyl group (-COOH), as their functional group. They are also known as carboxylic acids.
Alkanoic Formula
The general molecular formula is CnH2n+1COOH. The IUPAC name of each homologue is obtained by changing -e ending of the corresponding alkane to -oic acid.

General Properties of Alkanoic
- They are colourless at room temperature
- Their boiling point are much higher than expected when compared with their molecular size
- Alkanoic dissociate in aqueous solutions to produce hydrogen ions
- As the number of carbon atoms in the alkyl group increases, the acidic nature as well as the solubility of the alkanoic acids in water decreases
