Note On Specific Heat Capacity
Heat capacity is defined as the heat required to raise the temperature of the body through 1K. The unit is joules per Kelvin (J/K).
Specific heat capacity of a substance is the quantity of heat required to raise the temperature of unit mass (1kg) of the substance by 1K or 1oC. The S.I unit is J/kgk or J/kgC
The law of conservation of energy shows that the heat we supply does not just disappear. It is usually transformed into some other kind of energy. In this case, the heat supplied to the water is transformed to the internal energy of the water molecules. I shall focus on conservation principle of heat energy, H.C, S.H.C, and latent heat.
Read: difference between heat capacity and specific heat
Formula for Heat Capacity and Specific Heat Capacity
H.C= mass of substance x S.H.C
S.H.C = 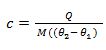
From the definition of S.H.C, it follows that
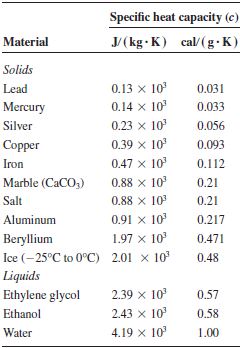
Heat capacity of 5kg of aluminium = Mass (5kg) x S.H.C of aluminium (910 J/kg.k) = 4550 J/K
or
H.C of 2kg of water = mass (2kg) x S.H.C of water (4200 J/kg.k) = 8400 J/K
Factors that does not affect specific heat capacity of a material
- It doesn’t depend on how much of the material is present.
- It’s also independent of the temperature interval i.e. if you double or tripple the teperature S.H.C is not affected
Specific Heat Capacities for some substances
Example1 Calculate the quantity of heat required to raise the temperature of 4kg of copper from 250C to 950C [Take S.H.C of copper = 390 J/Kgk]
Quantity of heat ![]()
M = 4kg, C = 390J/Kgk, ( = 95 – 25 =70
Q = 4 x 390×70
Q = 109200J
Determination of specific heat capacity of a solid
1. Method of Mixture
![]()
2. Electrical
![]()
Example 2: A piece of copper block of S.H.C 400J/kgk falls through a vertical distance of 20m from rest, calculate the rise in temperature of the copper block on hitting the ground when all its energies are converted into heat.
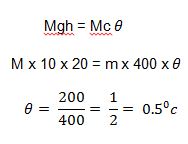
This potential energy is converted into kinetic energy as it falls and the kinetic energy to heat energy as it hits the ground.
Example 3: A heating coil is rated 75W, calculate the time it will take this coil to heat 1.4kg of water at 300C to 1000C (specific heat capacity of water =4200J/KgK)
Heat energy supplied by the heater = Heat absorbed by water
Ivt = Mcq
But power = IV,
Energy = Pt = Mcq
75 x t = 1.4 x 4200 x (100 – 30)
t = 54885 s
Change of state
Substance can exist in any three state of matter namely solid, liquid or gas. The state in which a substance exists depends on the temperature. Solid when heated change to liquid and when the liquid is further heated, it changes to gas. During change of state temperature remain constant.
Read: Differences between boiling and evaporation
Q = ML (Joule)
Where L is called the specific latent heat
L = Q/M = J/kg
Example 4: Calculate the amount of heat energy required to change 20kg of ice water at 00C. Specific content heat of ice = 336 x 103 J/kg
Q = 20 x 366 x
Q= 732 x J
Q= 7.32 x J
Read:
