Temperature Measurement In Physics For WAEC and JAMB
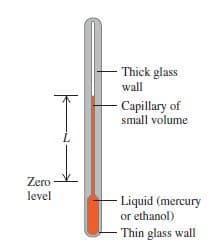
The idea of temperature is rooted in qualitative ideas of “hot” and “cold” based on the sense of touch. As we know an object that feels hot usually has a higher temperature than when it feels cold.
We are used to the idea that a thermometer shows the temperature of an object with which is in contact.
Temperature is a measure of the degree of hotness or coldness of an object on some scale. You can also say it tells us the direction in which energy flows (energy flows from a region of higher temperature to a region of lower temperature).
When two objects in contact have the same temperature, there will be no transfer of thermal energy between them. This is referred to as thermal equilibrium.
The unit of temperature includes Celsius, Kelvin, and Fahrenheit. However, the S.I base unit of temperature is Kelvin. Measuring temperature in Kelvin scale is better than that of Celsius scale in that one of its fixed points, absolute zero has much significance than the higher or lower fixed points of Celsius scale.
Note: it is impossible to have a temperature lower than zero Kelvin (0 K is absolute zero).
Read: 50, 30, 60, 40, 45, 100 Celsius to Fahrenheit
Conversion of different temperature scales
To convert from Celsius to Kelvin = temperature in Celsius + 273.15
To convert from Kelvin to Celsius = temperature in Kelvin – 273.15
Example
Convert -23 degree Celsius to Kelvin
Solution
-23 + 273.15 = 250.15 K
Note: the triple point of water is the temperature at which ice, water, and water vapour can co-exist. And it is defined as 273.16 K (273.16 – 273.15 = 0.01 C)
To convert from Celsius to Fahrenheit or vice-versa
C/5 = (F-32)/9
C is Celsius scale while F is Fahrenheit scale
Example
Convert 50 degree Celsius to Fahrenheit
50/5 = (F-32)/9
10 = (F-32)/9
10 X 9 = F-32
90 = F -32
F = 90 + 32
F = 122
