How to solve questions on Nuclear Physics for Cambridge A level
How to solve questions on Nuclear Physics for Cambridge A level
Question 1
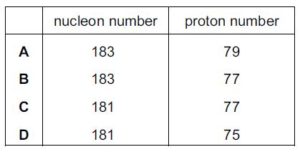
The gold nucleus 185Au79 undergoes alpha decay. What are the nucleon number and proton number of the nucleus formed by this decay?{cambridge A level may/june 2016 p12}
Solution
Au undergoes alpha decay
185Au79 – 181X77 + 4He2
The above equation is conserved since the number of nucleon and proton are the same before and after decay
The nucleon numberof the nucleus formed is 181 and the proton number is 77
C is the correct option
Question 2
In a time of 42.0 minutes, the count rate from the sample of copper-66 is found to decrease from 3.62 × 104 Bq to 1.21 × 102 Bq. Calculate the half-life of copper-66.{cambridge A level may/june 2016 p41}
Solution
![]()
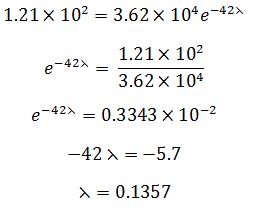
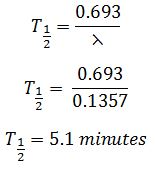
Question 3
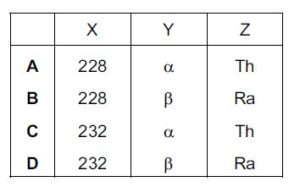
The diagram shows part of a radioactive decay chain in which the nuclide thorium-232 decays by α-emission into radium-228. This nuclide is also unstable and decays by β-emission into a nuclide of actinium. This process continues.{cambridge A leve oct/nov 2015 p11}
![]()
What are X, Y and Z?
Solution
since Ra undergoes beta decay X will still be 228: beta decay does not affect nucleon number
Y is beta since Th nucleon number isnt affect and the proton increase by 1
Z is Ra since the proton number is 88, after Th has undergone alpha decay, having the same proton number with Ra making it an isotope of Ra
B is the correct option
Question 4
In a model of a copper atom of the isotope 63Cu29, the atom and its nucleus are assumed to be spherical. The diameter of the nucleus is 2.8 × 10–14 m. The diameter of the atom is 2.3 × 10–10 m.{cambridge A leve oct/nov 2015 p22}
Calculate the ratio

Solution
denisty of nucleus = mass of nucleus / volume of nucleus
volume of nucleus = 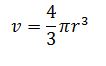
Volume = 4/3*3.142*(2.8×10^-14)^3
mass of nucleus = 63u
density of atom = mass of atom / volume of atom
volume = 4/3*3.142*(2.3×10^-10)^3
mass of atom = 63u
mass of atom and mass of nucleus (approx.) equal
ratio

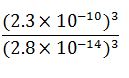
ratio = 5.5 x 10 ^11
Question 5
In the D-T reaction, a deuterium (2H1) nucleus fuses with a tritium (3H1) nucleus to form ahelium-4 (4He2) nucleus. The nuclear equation for the reaction iscambridge A level 2014 p41}
![]()
Some data for this reaction are given in Figure below
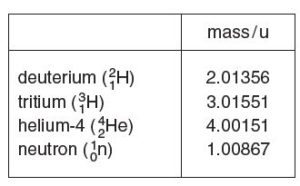
Use data from Figure above to determine the energy released in this D-T reaction.
Solution
total mass of the reactant = 2.01356 + 3.01551 = 5.02907 u
total mass of the product = 4.00151 + 1.00867 = 5.01018 u
mass defect = total mass of reactant – total mass of product = 5.02907 u – 5.01018 u
mass defect = 0.01889 u
note
1u is equivalent to 934 Mev
so,
0.01889 u = (933 * 0.01889) Mev
energy released in this D-T reaction = (934 * 0.01889) Mev = 17.6 Mev
Recommended: Question and answer on Nuclear for UTME
Recommended: Short notes on Nuclear physics
