Anomalous Behaviour of Water Explained With Questions
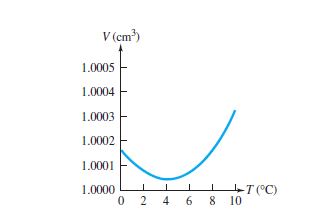
Water behaves anomalously when compared with other liquids at certain temperatures. Most liquid expand when heated and contract when cooled. However, at 0oc and 4o c anomalous behaviour of water occurs between those ranges of temperature.
When water is heated from 0oc to 4o c, it contracts and expands when cooled from 4oc to 0o c. But at any other temperature it behaves normal. This anomalous expansion of water makes ponds, lakes or rivers to freeze from the top surface rather from the bottom.
This anomalous behaviour of water also implies that when water is heated from 0oc to 4o c the volume decreases while the density increases.

In this range (0oc and 4o c), its coefficient of volume expansion is negative. Thus, water has its greatest density at 4o c. Water also expands when it freezes; that’s why pipes full of water burst if allowed to freeze. Most other materials contract when they freeze.
Mathematically,
Density = mass/volume (the higher the volume the lower the density and vice-versa).
Increase in inter-atomic spacing explains why liquids are generally less dense than solids.
The molecules of liquids have small spacing but with some gaps, less well ordered, and motion fairly slow.
Note: as the temperature of liquid rises from 0oc to 100oc, the molecules move increasingly rapidly. Their kinetic energy is increasing. There is very little change in the mean separation between the molecules and therefore very little change in their electrical potential energy.
Questions and Answer
1) At 40C, the volume of a fixed mass of water is
A. constant B. minimum C. maximum D. zero.
Answer
At 40C the volume of water is minimum.
2) If a container is filled with ice to the brim, what happens to the level of water when the ice completely melts?
A. The water in the glass outflows.
B. The level of water drops.
C. The level of water remains unchanged
D. The level of water goes up.
Answer
Option B.
Water expands on solidification (freezing); when this solid melts, it implies the water reduces in volume.
Read:
