Physic Tutorials
Simplifying physics for better understanding for students to excel in ‘A level’ and UTME
-
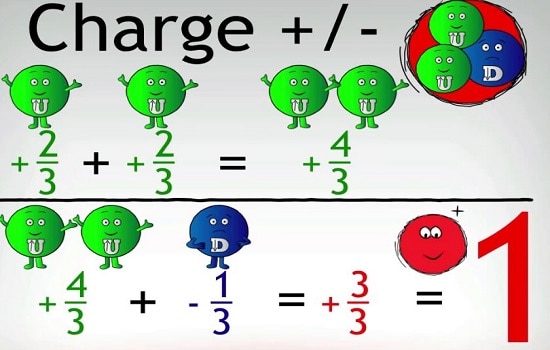
Nuclear Physics: Short Note on Quarks and Leptons
Quarks are the fundamental particles. They are what make up nucleons, i.e. protons and neutrons, as well as other particles.…
Read More » -

How to learn physics and tips to remember physics concepts
This article will guide you on how to learn physics and be good at it. Physics is one of the…
Read More » -

CIE A Level Physics Past Papers Solutions (Paper 1)
Cambridge A-level physics is easy to pass with grade A or A*, if you can work hard and be smart.…
Read More » -

Linear And Angular Kinematics Equations With Graphs
In this article, I will show the kinematic equations for both linear and angular, how they are derived, and graphs.…
Read More » -
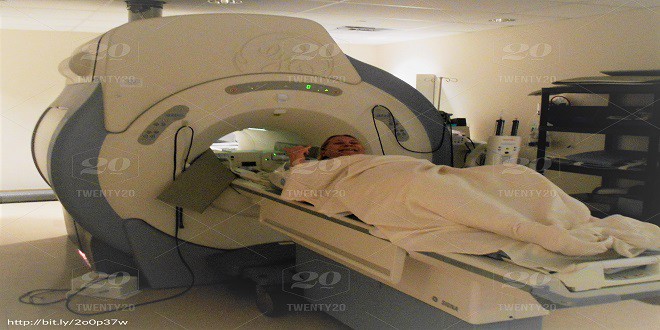
Magnetic Resonance Imaging (MRI operation and uses)
Magnetic resonance imaging, MRI is a diagnostic technique used in medicine. It can provide images of the inside of a…
Read More » -
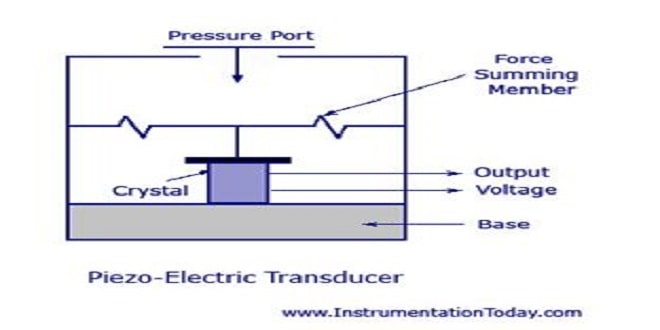
Ultrasound: Uses, Operation and how it is generated
Ultrasound is an oscillating sound pressure wave with a frequency greater than the upper limit of the human hearing range(…
Read More » -

Free download CIE Physics 9702 Past Papers
The page provides all the CIE Physics 9702 past papers. To have A* in Cambridge A level CIE physics isn’t…
Read More » -
How to solve Questions on Nuclear physics for UTME
How to solve Questions on Nuclear physics for UTME Question 1 A piece of radioactive material contains 1000 atoms. If…
Read More » -
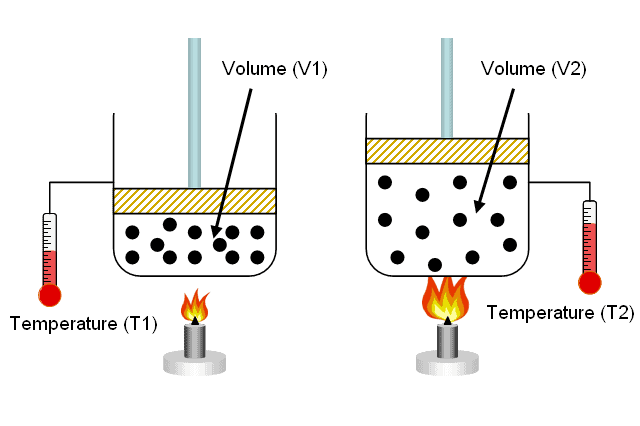
How to solve questions on gas law for UTME
How to solve questions on gas law for UTME Question 1 The pressure of a given mass of a gas…
Read More » -

Short notes on gas law for UTME
Short notes on gas law for UTME Boyle’s law Boyle’s law states that the volume of a fixed mass of…
Read More »
