Thermos Flask Diagram And Principles In Physics For WAEC & JAMB
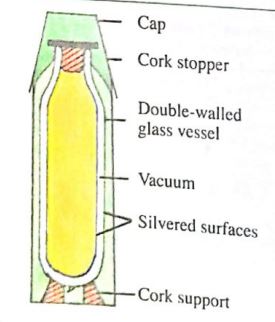
Thermos Flask is one of the applications of the three modes of heat transfer in physics. The principal features of the flask will be shown in the diagram below. Because of all the features in a thermos flask, heat loss or gained by the flask from the surroundings is very small. Hence the flask keeps cold liquid and hot liquids for a long time.
Diagram and Working Principles

The flask has a double-walled glass vessel with a vacuum between the walls. The vacuum is to reduce the loss or gain of heat by conduction or convection. The double walls of the glass vessel are silvered on the vacuum side. This minimizes the loss or gain of radiant heat to the outside.
The bottom of the flask rests on cork supports, and the mouth of the flask is also closed by a cork stopper or hollow plastic stopper with air inside. Cork, plastic, and air materials are all poor conductors; hence, no heat is lost or gained by conduction.
The cork stopper prevents heat loss by evaporation and convection if the flask contains hot liquid. The glass vessel is placed in an outer container made of plastic.
The above has explained the function of the key features of the thermos flask to reduce heat loss or gain.
Read: Thermal conductivity explained
This is one of the questions that students want to know, and I will answer in this article. The question is always in this form “ hot water in an ideal thermos flask is an example of ?”
From what I have explained above, hot water inside a thermos flask cannot change energy and matter with the environment. So, this made the flask an example of an isolated system. You know that in an isolated system, energy is conserved.
Also, in the flask, the heat energy remains constant. This makes it an adiabatic system.
Therefore, hot water in a thermos flask is an example of an isolated and adiabatic system.
