Surface Tension And Its Dimensional Formula
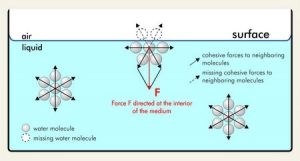
The surface of the liquid behave like a membrane under tension, surface tension arises because the molecules of the liquid exert attractive forces on each other. There is zero net force in a molecule inside the volume of the liquid, but a surface molecule is drawn into the volume. Thus, the liquid tend to minimise its surface area, just as a stretched membrane does.
Three examples of surface tension
- Water striders spend their life walking on water
- Surface tension causes the drops at the ends of these pipettes to adopt a near-spherical shape
- A paper clip rests on water, supported by surface tension
Surface Tension Dimensional Formula and Unit
Coefficient of surface tension is defined as the force per unit length acting in the surface at right angle to one sides of a line drawn in the surface. The s.i unit of surface tension is Nm-1 (Newton per meter).
Dimension
y = F/L
The dimension of force is MLT-2
The dimension of length is L
Y = MLT-2/L = MT-2
So the dimension of coefficient of surface tension is MT-2.
Adhesion and Cohesion
Cohesion is the force of attraction between molecules of the same kind. Adhesion is the force of attraction between molecules of different kind.
Cohesion and Adhesion explain the different action of water and mercury when spilled on a clean glass surface.
Water: The adhesion of water molecules to glass is stronger than the cohesion between water molecules, water spread out on a clean glass surface when sprinkled on it and wet the glass.
Mercury: The force of cohesion between two molecules of mercury is greater than the force of adhesion between a molecule of mercury and a molecule of glass; thus mercury gathers in pools when split on glass.
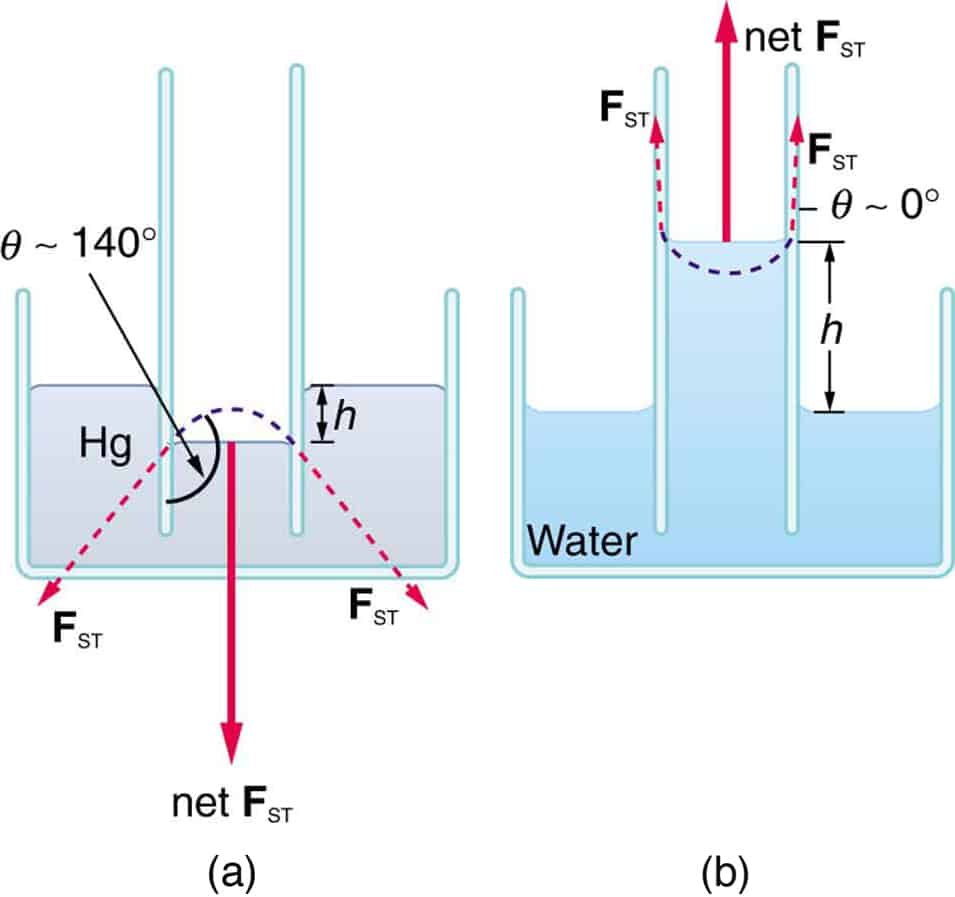
Angle of contact
The angle of contact is the angle between the tangent to the liquid surface and the tangent to the liquid surface, both drawn in the place at right angle to both surfaces and meeting at the point of contact measured within the liquid.
For a liquid in which the cohesive force is greater than the adhesive force, the angle of contact is obtuse e.g mercury. If the adhesive force exceeds the cohesive force of the liquid molecules, the angle of contact is very small or almost zero e.g water
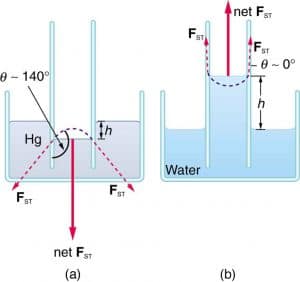
Capillarity
It is the tendency of a liquid to rise or fall in a capillarity tube. Cohesion and adhesion as well as surface tension forces are responsible for the capillarity of liquids. In water and soap solution the surface of the liquid or its meniscus curves upward. But in mercury the meniscus is curved downwardsmaway from the glass tube.
Capillarity explains why liquid candle wax rises up the wick of a candle or kerosene rises up the wick of a lamp.
Recommended: Solved questions on linear expansivity
